Determine Which Decay Process Is Being Described in Each Case.
In this case when comparing the masses on the two sides of the. A steady but unpredictable spontaneous process.

Pin By Rashmi Bohra On Sonibadhani Chemistry Paper Chemistry Past Papers
Writing nuclear equations for alpha beta and gamma decay.

. Determine the mean and the standard deviation and check that the relation σ n still seems valid. Next the features of the assay were tested. Half-life and carbon dating.
Rate law expression for first order kinetics is given by. Match each type of radioactive decay process with the correct description of the accompanying change in the nucleus. Each decay will emit either an a-particle or a β-particle and may be accompanied by γ-rays.
I really dont know where to start. When helium nuclei come flying off of the nucleus of a larger isotope forming an isotope with a smaller mass. Each of these modes of decay leads to the formation of.
A statistical process is described through a Poisson Distribution if. This process continues as a radioactive decay series until a stable nuclide is reached. Where k rate constant.
The decay equations and masses of the isotopes appear below. Z AX 1 Z1 AX 2 1 0eν e. Exponential decay and semi-log plots.
30-3a the minus sign means N decreases in time. Random process for a given nucleus probability for a decay to occur is the same in each time interval universal probability the probability to decay in a given time interval is same for all nuclei no correlation between two instances the decay of one. The proportionality constant A.
The validation process showed. Tap card to see definition. Experiment with the best choice of bin-size.
The probability to decaytime is termed the decay constant and is given the symbol λ. Radioactive decay is a statistical process. Click card to see definition.
General equation for beta-plus decay The neutrino in this case is an electron neutrino there are two other kinds of neutrino each with an antimatter version. All radioactive decay follows first order kinetics. There are three major types of radioactive decay.
λ the probabilty to decay per unit time units of 1time From this assumption one can derive the half-life decay rule as follows. Polonium-218 goes through a series of seven decays to become a stable lead-206 atom as shown in Figure. Knowing which system is appropriate.
The decay of each individual radioactive atom appears to be fundamentally deterministic. Nuclear reactions also often involve γ rays and some nuclei decay by electron capture. The decay of an unstable nucleus is entirely random in time so it is impossible to.
Beta-plus decay takes the nucleus one step down the periodic table. Again we find a chance process being described by an exponential decay law. The steps are the same as in the.
Process is unique and diagnostic for each targeted decay agent. The most common types of radioactivity are α decay β decay γ emission positron emission and electron capture. Each case violates one of Everetts goals.
Radioactivity and radioactive decay are spontaneous processes. Expression for calculating half life which is the time taken by the half of the reactants to decompose is. A initial amount of the reactant.
Exponential decay formula proof can skip involves calculus Introduction to exponential decay. Mother - daughter equations for alpha beta or gamma. Alpha decay involves the loss of a helium nucleus beta decay concerns protons turning into neutrons or vice versa and gamma decay involves the emission of energy without changing the original atom.
A - x amount left after decay process. The decay process can follow either a radiative path ie emission of X-ray photons of energies hω and intensities I R h ω that constitute the fluorescence spectrum or a nonradiative path ie autoionization after core-excitation and Auger decay after core-ionization with emission of Auger electrons of characteristic energies ε j A and intensities I A ε j A that constitute the. Consider the case of a nuclide A that decays into another B by some process A B emission of other particles like electron neutrinos ν e and electrons e as in beta decay are irrelevant in what follows.
I have a few problems to prove like this but no examples. More exponential decay examples. Describe why you use the choice of bin-size that you do.
You should find good agreement between the calculated Q and the given E M for the Tl-204. We can easily find an expression for the chance that a radioactive atom will survive be an original element atom to at least a time t. Respect to the decay process being observed 4 5 before.
This was done not only by testing the assay on fungal fruit bodies and fungal cultures but also by testing wood from trees characterized either by failures or by the presence of obvious decay symptoms. For a given type of radioactive nucleus the number of nuclei that decay AN in a time At is proportional to the number N of parent nuclei present. Given maximum beta decay energies.
Determine if decay of U A-236 Z-92 - U A-235 Z-92 n is possible. T time taken for decay process. The value of the decay constant depends on the nature of the particular decay process.
Differential calculus is used to model the behaviour of nuclear decay. However you should find that the Q calculated for Co-60 is significantly greater than the given E. Recall that Q Δm c 2 where Δm is the mass deficit in the decay process.
The spontaneity or randomness of radioactive decay the reason isotopes are important the concept of half-life and. Again you might also find it useful to use a spreadsheet. Alpha decay beta decay and gamma decay.
Then plot the expected theoretical distribution for this mean value and. I describe some recent versions of. Where the quantity l known as the radioactive decay constant depends on the particular radioactive substance.
Positron emission matches ------------- The number of protons in the nucleus decreases by 1 while the number of neutrons increases by 1. I understand how to caluclate the binding energy and how much energy is released but I dont understand. Predictable random however contrary to the view that nature is Immanuel Kant - Time is a Mental Construct Immanuel Kant 1774-1804 held that time and other conceptual categories such as causation number spacial relations and so on are built-in categories and filters like.

Dental Implant Timeline From Start To Finish Have More Questions Call Us Dentalimplants I Dental Implants Cost Dental Implants Dental Implants Infographic

How Do We Know The Earth Is Old Earth And Space Science Teaching Science Earth

Sisu Definition Print Finnish Wall Art Living Room Decor Etsy Motivational Prints Inspirational Quote Prints Art Quotes Inspirational

5 Tips To Help You Get Ready For Dental Visit Www Unitedsmiles Com Au Dental Posts Dental Dental Problems

Pin By Audrey Rhunette On Study Motivation School Study Tips School Organization Notes Notes Inspiration
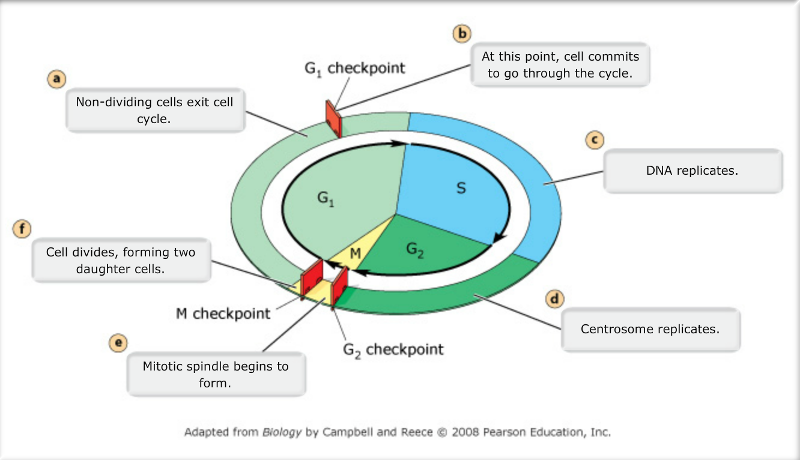
Interphase 90 Cell Cycle Biology Notes Cell Cycle Biology

Pin On Cg Lighting Post Process Rendering

Infographic Analytics In The Digital Age How To Measure The Effectiveness Of Marketing With Big Data Marketing Measurement Measuring Success Infographic

Individuation Hero S Journey Writing Plot Writing A Book

Iphone Wallpaper Gold Wallpaper Iphone Gold Wallpaper Iphone Wallpaper

Variance And Standard Deviation Exercise Doc Standard Deviation Data Science Learning Worksheets

Greatest Common Factor Of A Polynomial Google Classroom Distance Learning Distance Learning Algebra Activities Google Classroom

Eileen Gray Thoroughly Modern Maker Eileen Gray Eileen Gray Furniture Furniture Design Table

Algebra 1 Solving Quadratic Functions Finding Roots Flip Book Quadratics Algebra 1 Algebra

The Musical Arts Music Musician Paintings Francois Guiguet La Violoniste 1914 Violin Art Musical Art Music Art

An Experience That Taught You A Valuable Lesson About Life Essay Example Essay Examples Essay About Life Essay



Comments
Post a Comment